Generic Availability: Why the Same Drug Costs Different Amounts Around the World
 Jan, 25 2026
Jan, 25 2026
Ever bought a prescription drug in one country, then traveled to another and found the same pill at half the price-or worse, couldn’t find it at all? It’s not a glitch. It’s the global generic drug market, and it works nothing like you’d expect. The same active ingredient, made by the same factory, sometimes sold under the same brand name, can cost 600% more in one country than another. And in some places, doctors won’t even prescribe it. Why?
Same Drug, Different Rules
Generic drugs are supposed to be identical to brand-name versions-same active ingredient, same dose, same effect. But the rules around what counts as "identical" vary wildly. The U.S. Food and Drug Administration (FDA) requires generics to match the brand-name drug’s absorption rate within 80-125%. The European Medicines Agency (EMA) uses a similar range, but how they test it, where they inspect factories, and how often they check for quality differ. In the U.S., inspections of foreign manufacturing sites are often unannounced. In Europe, many are scheduled months in advance. That gap matters. A 2023 study from Ohio State University found that generic drugs made in India had 54% higher rates of severe adverse events-including hospitalizations and deaths-compared to the same drugs made in the U.S. The difference? Not the chemical. The manufacturing control.Who Gets Generic Drugs-and Who Doesn’t
In the United Kingdom, 83% of all prescriptions are filled with generics. In Switzerland, it’s 17%. That’s not because British patients are more trusting or Swiss doctors are more loyal to brands. It’s policy. The UK forces pharmacies to substitute generics unless a doctor specifically writes "do not substitute." Germany and the Netherlands do the same. In Switzerland, doctors are reimbursed more for prescribing the original brand. So they do. In Italy and Greece, generics make up just 20% of prescriptions-not because they’re unsafe, but because the system doesn’t push them. In the U.S., 90% of prescriptions are for generics. Sounds great, right? But here’s the twist: the U.S. pays more for generics than almost any other country. The average price for a 30-day supply of generic metformin? Around $10 in the UK. In the U.S., it’s $25. In Canada, $12. In India, $1. The FDA approves hundreds of generic versions of the same drug. But if only one manufacturer is producing it, and the others went out of business because prices got too low, that one company can raise prices overnight. That’s what happened with doxycycline, cyclosporine, and dozens of others. The system meant to save money ended up creating dangerous monopolies.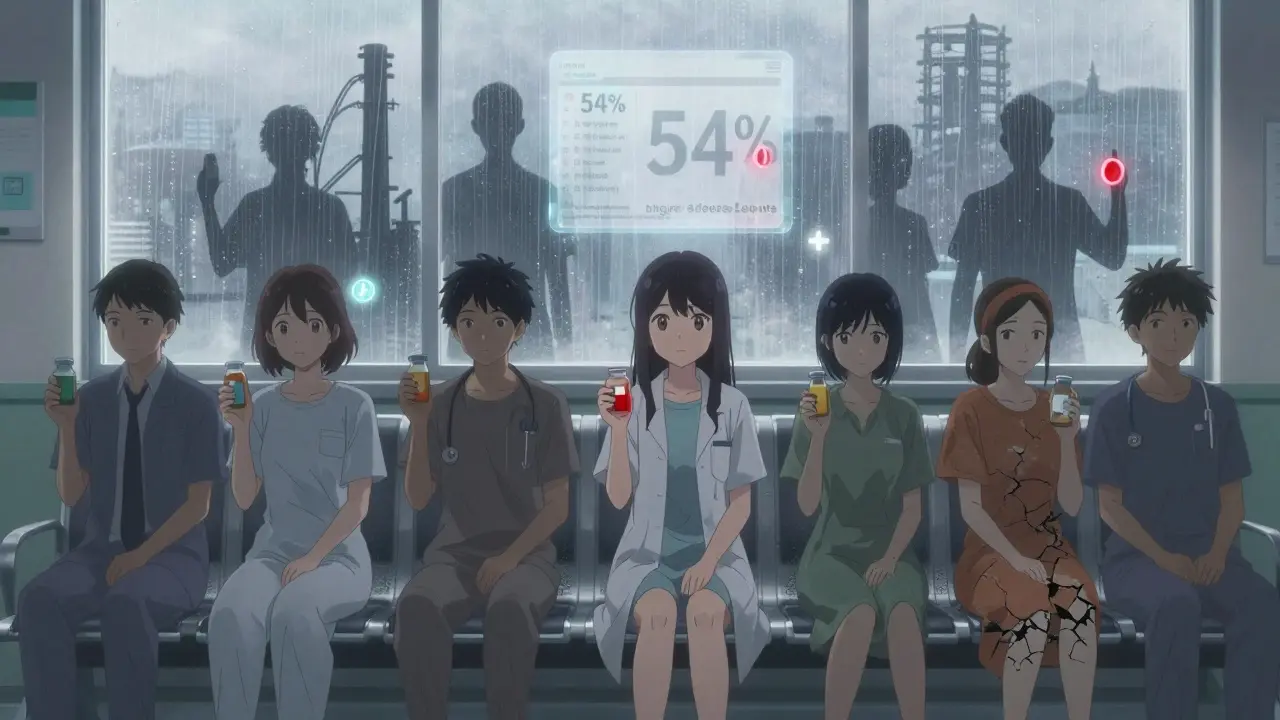
The Indian Factory Effect
India makes 40% of the generic drugs used in the U.S. and 20% of the world’s total supply. Over 750 Indian manufacturing plants are FDA-approved. That’s more than any other country. But approval doesn’t mean safety. A 2023 FDA report found that 37% of inspection violations for foreign drug plants came from India. Many of these plants are under pressure to cut costs. When a drug’s patent expires and 20 companies start making it, the price drops fast. To survive, manufacturers reduce quality control. They use cheaper fillers. They skip stability tests. They cut corners on cleaning between batches. That’s why some patients report different side effects when switching from a U.S.-made generic to an Indian-made one-even if both are labeled the same. Cipla, one of India’s biggest generic makers, has 78% positive reviews from U.S. customers on PharmacyChecker. But 22% say their blood pressure spiked, their thyroid levels went haywire, or they got dizzy after switching. It’s not always the drug. It’s the excipients-the inactive ingredients. One brand might use cornstarch. Another uses lactose. If you’re allergic to one, you won’t know unless you check the label. And in many countries, those labels aren’t translated or standardized.Why the U.S. Pays More for More Generics
The U.S. is the world’s largest market for generic drugs-and the most expensive. Why? Because it doesn’t negotiate prices. Medicare can’t bargain with drugmakers. Private insurers don’t have enough leverage. So even when 15 companies make the same generic, they all charge the same high price. That’s called price collusion by default. No one lowers their price because no one has to. In contrast, in the UK, the National Health Service sets a single price for each generic. Everyone gets paid that amount. If you want to make money, you have to make it cheaper than the next guy. That forces efficiency. In the U.S., it forces consolidation. And when only one company is left making a drug? That’s when shortages hit. In 2023, the FDA recorded 147 generic drug shortages. Two-thirds were linked to manufacturing quality issues-mostly in India and China. One plant shut down because of mold in the production room. Another because the water system failed. The drug? A common antibiotic. Patients went without. Doctors scrambled. The system wasn’t broken. It was designed this way.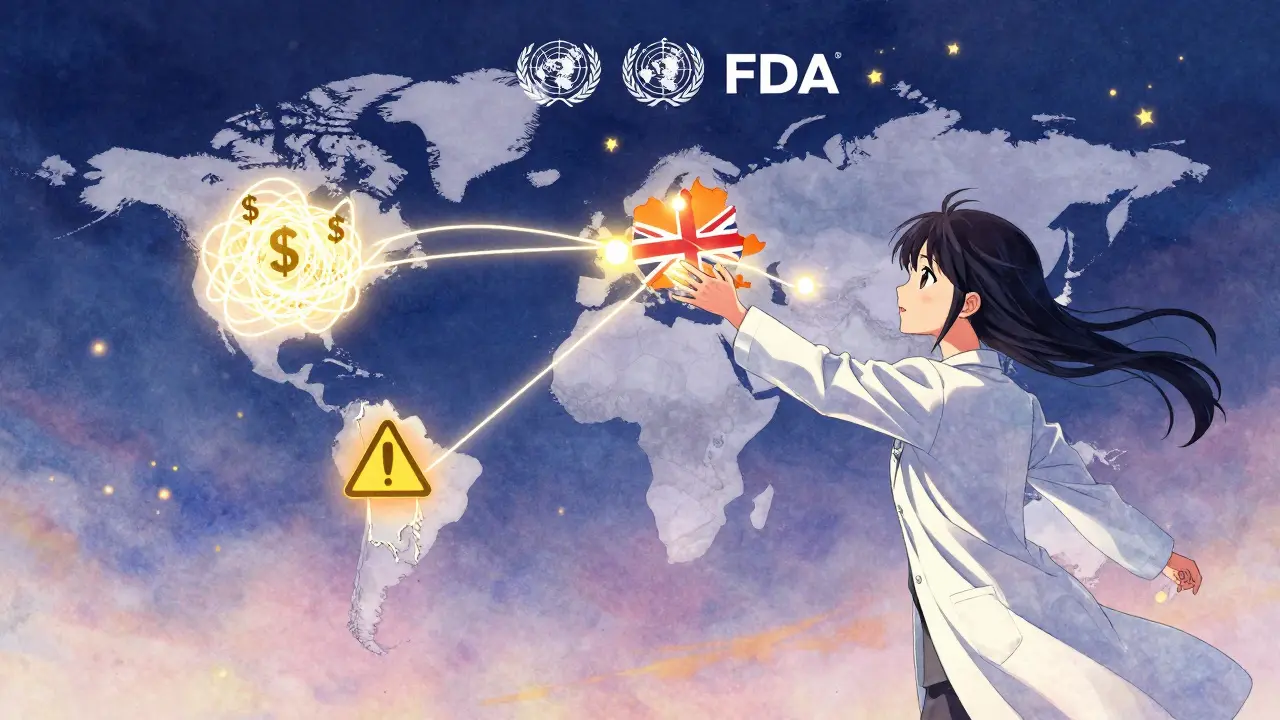
What’s Changing-and What’s Not
The European Union is trying to fix this. Its 2023 Pharmaceutical Strategy wants all member states to hit 80% generic use by 2030. It’s pushing for shared inspections, common labeling, and faster approval across borders. The U.S. Inflation Reduction Act of 2022 gave the FDA more money to inspect foreign plants and cut review times by 30%. The World Health Organization launched a new global benchmarking tool to force countries to prove their generic quality controls are real. But the biggest barrier isn’t technical. It’s political. Every country wants to protect its own drugmakers. The U.S. wants cheap imports but won’t let foreign regulators approve its own plants. Europe wants uniformity but lets each nation set its own reimbursement rates. India wants to export more but won’t tighten its standards unless forced. China is building factories faster than anyone-but the FDA still flags half its inspections for violations.What This Means for You
If you’re on a generic drug, here’s what you need to know:- Don’t assume all generics are the same. If you switch brands and feel different-dizzy, nauseous, your condition worsens-tell your doctor. It’s not "all in your head."
- Check the manufacturer. Look at the pill imprint or ask your pharmacist. If you’ve been on one made in the U.S. and suddenly get one from India, ask why. You have a right to know.
- Don’t buy from unverified online pharmacies. Some sites sell Indian generics at 80% off. But they might be expired, counterfeit, or stored in hot warehouses. The FDA warns about this constantly.
- Know your country’s rules. If you travel, bring your prescription and a list of the generic names. In some countries, your U.S. generic might not be available-or might be a different salt form entirely.
There’s no global standard for what makes a generic safe. There’s no global price. There’s no global trust. What you get depends on where you live, who makes it, and who’s paying for it. The system was built to save money. But without real oversight, it’s just a gamble.
Why are generic drugs cheaper if they’re the same as brand-name drugs?
Generics are cheaper because they don’t pay for research, marketing, or advertising. The original drug company spent billions developing the drug and running clinical trials. Once the patent expires, other companies can copy the formula without those costs. They just need to prove it works the same way. That saves money-but doesn’t always mean better quality.
Can I trust generics made in India or China?
Many are safe-FDA-approved factories in India and China produce the majority of the world’s generics. But inspections are inconsistent. Some plants meet high standards. Others cut corners to save money. A 2023 study found Indian-made generics had 54% higher rates of severe side effects than U.S.-made ones. If you notice new side effects after switching to a foreign-made generic, talk to your doctor. Don’t assume it’s your body adjusting.
Why does the U.S. use so many generics but still have high drug prices?
The U.S. has high generic use because pharmacies are required to substitute them. But prices aren’t negotiated. When multiple companies make the same drug, they often charge the same high price instead of competing. If one company stops making it, the rest can raise prices. That’s why some generics cost more in the U.S. than in Canada or the UK-even though they’re the same pill.
Are there countries where generics aren’t available at all?
Not completely, but in places like Switzerland and Italy, generics are rare. Doctors there are often paid more to prescribe the original brand. Patients are taught to trust brand names. Even when generics exist, they’re rarely used. In these countries, it’s not about availability-it’s about culture, reimbursement rules, and how doctors are incentivized.
What’s being done to fix global generic drug disparities?
The WHO, FDA, and European Commission are pushing for shared inspection standards, faster approvals, and better labeling. The U.S. Inflation Reduction Act is increasing funding for FDA inspections. The EU wants all countries to hit 80% generic use by 2030. But progress is slow. Countries protect their own systems. Without global enforcement, quality and pricing will stay uneven.

Robin Van Emous
January 27, 2026 AT 20:48Wow, I never realized how much the same pill could vary just based on where it was made. I’ve been on generic metformin for years and never thought to check the manufacturer. Now I’m kinda paranoid every time my pharmacy switches it up.
My cousin in India got the same drug for $0.50 a month-same batch number, same packaging. But she said her stomach acted up after switching. Turns out the filler was different. Who knew cornstarch could be a trigger?
This isn’t just about money. It’s about trust. And right now, the system’s broken.
I’m not anti-generic. I’m pro-transparency. If I’m swallowing something that’s supposed to keep me alive, I deserve to know what’s in it-and who made it.
rasna saha
January 28, 2026 AT 17:04As someone from India, I see this every day. Our factories are working nonstop to supply the world, but the pressure? Unreal.
We’re not cutting corners because we’re lazy-we’re cutting because the bids are so low, we can’t survive otherwise. A single inspection violation can shut us down for months.
But let’s be real-some of us still care. My uncle works in a plant that does full stability tests, even when no one’s watching. He says, ‘If it’s not safe for my daughter, it’s not safe for yours.’
We need better pricing, not just more inspections. Quality shouldn’t be a luxury.
Skye Kooyman
January 28, 2026 AT 21:51So the U.S. pays more for generics because no one’s negotiating? That’s wild.
Also, mold in a production room? That’s not a glitch. That’s a crime.
James Nicoll
January 29, 2026 AT 21:33Let me get this straight. The U.S. has the most generics, the worst prices, and the least oversight. And we call this capitalism?
Meanwhile, the UK just says ‘here’s the price, make it cheaper or go home.’ And somehow, no one dies.
Maybe we should stop pretending this is a free market and start calling it a cartel with a side of placebo.
Peter Sharplin
January 30, 2026 AT 07:11There’s a huge difference between ‘approved’ and ‘safe.’ The FDA approves based on paperwork and occasional visits. But quality control? That’s daily. It’s the people in the factory, the temperature logs, the cleaning schedules, the training.
One plant I visited in Hyderabad had a 30-second rule: if you’re not scrubbing your hands for 30 seconds before entering the clean room, you’re not coming in. That’s the kind of detail that saves lives.
But when you’re pressured to produce 10 million tablets a day at $0.02 each? That rule gets ignored. Not because they’re evil. Because they’re desperate.
Patients need to know: if your blood pressure spikes after switching generics, it’s not psychosomatic. It’s chemistry. And someone’s cutting corners.
Also-check the pill imprint. You’d be shocked how many ‘generic’ pills are actually knockoffs sold online. The FDA has a database. Use it.
shivam utkresth
January 31, 2026 AT 04:19Bro, we’re the pharmacy of the world and nobody gets it.
Our factories? They’re not some shady back-alley ops. We’ve got ISO-certified, GMP-compliant, FDA-approved plants that could run circles around half the U.S. facilities.
But then the U.S. comes in with a $0.01 bid and says ‘make it cheaper or we’re going to Canada.’ So we drop the excipient cost, skip a batch test, use cheaper packaging-just to survive.
And then we get called ‘unsafe’? Come on.
It’s not us. It’s the system. We’re just the ones holding the bag while the West buys cheap and blames us when it breaks.
John Wippler
February 1, 2026 AT 23:57Here’s the truth nobody wants to admit: the generic drug system was never meant to be fair. It was meant to be cheap. And cheap doesn’t mean safe. It means ‘barely acceptable.’
We treat medicine like a commodity, not a lifeline. We let corporations optimize for profit, not patient outcomes. And then we act surprised when people get sick.
Imagine if your car’s brake pads were made by the lowest bidder, and you had no idea who made them or where they came from. You’d never drive.
So why do we drive with pills?
Kipper Pickens
February 2, 2026 AT 10:00Let’s unpack the regulatory arbitrage here. The FDA’s 80-125% bioequivalence window is a legal loophole disguised as science. Two drugs can be pharmacokinetically divergent by 45% and still be deemed ‘interchangeable.’
Meanwhile, EMA’s reference-scaled average bioequivalence (RSABE) approach is statistically more robust, but politically inconvenient.
And don’t get me started on excipient heterogeneity. Lactose vs. cornstarch isn’t just a ‘sensitive stomach’ issue-it’s an immunological variable in chronic disease populations.
This isn’t a supply chain problem. It’s a governance failure.
Aurelie L.
February 3, 2026 AT 12:39My mum had a stroke because her blood thinner changed. She didn’t know. The pharmacy switched it. She died before they figured out why.
Don’t tell me this is just ‘a pricing issue.’ It’s a murder-by-clipboard.
Faisal Mohamed
February 3, 2026 AT 20:07So… we’re basically playing global pharmaceutical Russian roulette? 🤡
One pill from India, one from Germany, same box, same name, different outcomes. And we’re supposed to just ‘trust the system’? Nah.
Bring on the global FDA. Or at least a barcode that tells me who made it. 🙏
SWAPNIL SIDAM
February 5, 2026 AT 05:53From India to the U.S.-I’ve seen both sides. My brother works in a U.S. pharmacy. He says they don’t even know which generic they’re getting until the box arrives.
One day it’s from Ohio. Next week, it’s from Bangalore. No warning. No label change.
People get dizzy. They think it’s stress. It’s not. It’s chemistry. And nobody’s telling them.
Someone needs to make this visible. Like a food label. ‘Made in India. May cause dizziness.’
Sally Dalton
February 6, 2026 AT 07:27omg i just checked my metformin and it says ‘Sun Pharma’-i thought it was made in the us 😭 i’ve been on this for 5 years and never looked. i think i’ve been having weird headaches since last month… should i tell my dr? i dont want to sound crazy
Betty Bomber
February 6, 2026 AT 11:58My dad’s on blood pressure meds. Switched generics last year. Now he’s dizzy all day. Doctor said ‘it’s just aging.’
Turns out, the new one had a different filler. He’s back on the old one now.
Just… check your pills. Please.